- +1-315-215-1633
- sales@thebrainyinsights.com

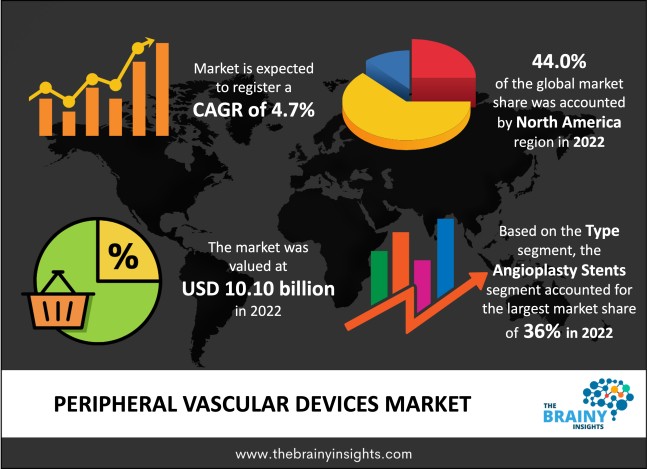
The global peripheral vascular devices market was valued at USD 10.10 billion in 2022 and growing at a CAGR of 4.7% from 2023 to 2032. The human circulatory system comprising arteries, veins, and capillaries, is vital for ensuring the overall healthiness of a human being because the primary focus of the circulatory system is the more accessible transportation of essential nutrients and oxygen to be transported throughout the body. The circulatory system also takes away the waste products of the human body. Peripheral vascular disorders (PVDs), which include a variety of disorders that affect these vessels, can severely negatively affect a person's quality of life. As a result, the invention of peripheral vascular devices has significantly developed medical and healthcare technology. These devices diagnose, analyze, and treat peripheral vascular disorders, improving circulatory health and promoting general well-being.
Peripheral vascular devices offer treatments for various circulatory system problems affecting the arteries, veins, and capillaries away from the heart and brain, and they represent a unique convergence of medical innovation and technology. Peripheral vascular diseases (PVDs), a broad category of illnesses that can result in reduced blood flow, tissue damage, and even life threatening complications, cover many problems. The primary goal of the peripheral vascular devices industry is to develop advanced instruments and technologies that assist in identifying, managing, and treating these conditions. These advancements will ultimately restore blood flow, ease symptoms, and enhance patients' quality of life. Accurate diagnosis is the cornerstone of any medical intervention that works. Diagnostic tools for peripheral vascular disease have transformed how medical professionals evaluate the condition of blood vessels. These devices use advanced technologies to provide accurate insights into the type and severity of vascular diseases. Non-invasive techniques like Doppler ultrasound enable real-time blood flow visualization, assisting in detecting vascular anomalies such as stenosis and occlusion. Clinical decision-making is made possible by using advanced imaging techniques like computed tomography angiography (CTA) and magnetic resonance angiography (MRA), which provide high-resolution images of blood arteries.
Get an overview of this study by requesting a free sample
Increasing Prevalence of Cardiovascular Diseases- Smoking, unhealthy eating practices, and drastic lifestyle changes significantly contribute to the rise in PVDs. People are seeking early interventions to manage their cardiovascular health as awareness of these risk factors and the importance of preventive health care increase. Higher demand for peripheral vascular devices that can diagnose and treat these illnesses in their early stages has resulted from this change in healthcare-seeking behavior. As a result, the market has undergone a surge fueled by patient awareness and the desire to embrace healthier lifestyles. Lower extremity peripheral artery disease (PAD) impacts over 220 million individuals globally. It is correlated to a risen risk of several unfavorable clinical results (consisting of cardiovascular diseases like stroke and limb outcomes such as amputation, as well as coronary heart disease), according to the scientific study titled 'Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions' and published by the American Heart Association in August 2021. This rising incidence of PAD is expected to boost the demand for peripheral vascular devices in upcoming years.
Device Costs and Procedure-related Expenses- Innovative peripheral vascular devices frequently need complex engineering, advanced materials, and cutting-edge technologies to develop. These elements help to explain why production, research, and regulatory compliance have high prices. As a result, the cost of these devices at first purchase can be high. The significant investment necessary to acquire these devices can put a burden on funding for healthcare organizations, particularly in settings with limited resources. It may ultimately limit their ability to provide patients with advanced medical treatments. Peripheral vascular procedures come with various expenses that affect patients, healthcare providers, and institutions in addition to the devices' costs. These expenses cover the price of the operating room staff, anesthesia, and surgical facilities. The complexity of these procedures frequently necessitates specialized equipment and an experienced medical team, which increases the overall costs. Thus, this factor is hindering the market growth and development.
Technological Advancements- In the present scenario, technology has become essential for organizations engaged in the medical and healthcare industry and is continuously gaining momentum. The ongoing technological developments and advancements within the field of peripheral vascular devices are anticipated to provide lucrative growth opportunities in the upcoming years. The domain of peripheral vascular devices has changed due to advances in materials science, imaging techniques, and less invasive procedures. With the aid of imaging technologies like magnetic resonance angiography (MRA), computed tomography angiography (CTA), and intravascular ultrasound (IVUS), healthcare professionals may now see vascular structures with previously unheard-of clarity. Advances have greatly improved the treatment outcomes in stain design, drug-eluting coatings, and bioresorbable materials, decreasing complications and accelerating patient recovery. Additionally, developing healthcare infrastructure, especially in emerging economies, has been crucial to the market growth for peripheral vascular devices. A more extensive section of the population has access to advanced medical interventions as healthcare systems become more advanced and accessible.
The regions analyzed for the market include Europe, North America, South America, Asia Pacific, and Middle East & Africa. North America emerged as the largest market for the global peripheral vascular devices market, with a 44.0% share of the market revenue in 2022. The regional market growth is mainly attributed to advanced medical systems/technologies and robust healthcare infrastructure. Furthermore, the rise in cases of cardiovascular diseases, especially in the countries such as the U.S., is expected to drive the demand for peripheral vascular devices in the region. Additionally, the regional market players engage in various market strategies such as mergers, acquisitions, partnerships, and strategic alliances to maintain their competitive edge.
North America Region Peripheral Vascular Devices Market Share in 2022 - 44.0%
www.thebrainyinsights.com
Check the geographical analysis of this market by requesting a free sample
The type segment is divided into angioplasty balloons, angioplasty stents, catheters and others. The angioplasty stents segment dominated the market, with a market share of around 36% in 2022. Angioplasty stents are a type of peripheral vascular device with high safety, tolerability and efficiency. The U.S. Food and Drug Administration (FDA) recommends using Paclitaxel-coated balloons and stents to treat people who have femoropopliteal artery atherosclerotic lesions that are both new and recurrent. The stent functions to unblock the blood vessel mechanically. Zilver PTX and ELUVIA drug-eluting vascular stent systems, manufactured by Cook Ireland Ltd. and Boston Scientific Corporation, are two examples of peripheral stents with FDA approval. Further product developments and innovations within the category of stents are anticipated to fuel segment’s expansion.
The end-user segment is divided into hospital, ambulatory surgical centers and specialty clinics. The hospital segment dominated the market, with a market share of around 53% in 2022. Comprehensive medical care and medical treatments are primarily offered in hospitals. They have several specialized departments and sections, such as cardiology, radiology, and vascular surgery, which rely heavily on peripheral vascular devices. In addition, hospitals frequently have cutting-edge amenities, sophisticated diagnostic equipment, and a staff of highly qualified medical specialists. This enables them to manage challenging situations and perform treatments that call for peripheral vascular devices. The increased number of people seeking treatment in hospitals for various vascular problems as a result is what fuels the market for peripheral vascular devices. This is expected to impact the development of the global market positively.
| Attribute | Description |
|---|---|
| Market Size | Revenue (USD Billion) |
| Market size value in 2022 | USD 10.10 Billion |
| Market size value in 2032 | USD 16.00 Billion |
| CAGR (2023 to 2032) | 4.7% |
| Historical data | 2019-2021 |
| Base Year | 2022 |
| Forecast | 2023-2032 |
| Region | The regions analyzed for the market are Asia Pacific, Europe, South America, North America, and Middle East & Africa. Furthermore, the regions are further analyzed at the country level. |
| Segments | Type, End-user |
As per The Brainy Insights, the market size of the global peripheral vascular devices market was valued at USD 10.10 billion in 2022 to USD 16.00 billion by 2032.
Global peripheral vascular devices market is growing at a CAGR of 4.7% during the forecast period 2023-2032.
The market's growth will be influenced by the growing adoption of minimally invasive procedures in developed and developing countries.
The stringent regulatory challenges is anticipated to hamper the market growth.
1. INTRODUCTION
1.1. OBJECTIVES OF THE STUDY
1.2. MARKET DEFINITION
1.3. RESEARCH SCOPE
1.4. CURRENCY
1.5. KEY TARGET AUDIENCE
2. RESEARCH METHODOLOGY AND ASSUMPTIONS
3. EXECUTIVE SUMMARY
4. PREMIUM INSIGHTS
4.1. PORTER’S FIVE FORCES ANALYSIS
4.2. VALUE CHAIN ANALYSIS
4.3. TOP INVESTMENT POCKETS
4.3.1. MARKET ATTRACTIVENESS ANALYSIS BY TYPE
4.3.2. MARKET ATTRACTIVENESS ANALYSIS BY END-USER
4.3.3. MARKET ATTRACTIVENESS ANALYSIS BY REGION
4.4. INDUSTRY TRENDS
5. MARKET DYNAMICS
5.1. MARKET EVALUATION
5.2. DRIVERS
5.2.1. GROWING MINIMALLY INVASIVE PROCEDURES
5.3. RESTRAINTS
5.3.1. REGULATORY CHALLENGES
5.4. OPPORTUNITIES
5.4.1. TECHNOLOGICAL ADVANCEMENTS
6. GLOBAL PERIPHERAL VASCULAR DEVICES MARKET ANALYSIS AND FORECAST, BY TYPE
6.1. SEGMENT OVERVIEW
6.2. ANGIOPLASTY BALLOONS
6.3. ANGIOPLASTY STENTS
6.4. CATHETERS
6.5. OTHERS
7. GLOBAL PERIPHERAL VASCULAR DEVICES MARKET ANALYSIS AND FORECAST, BY END-USER
7.1. SEGMENT OVERVIEW
7.2. HOSPITALS
7.3. AMBULATORY SURGICAL CENTERS
7.4. SPECIALTY CLINICS
8. GLOBAL PERIPHERAL VASCULAR DEVICES MARKET ANALYSIS AND FORECAST, BY REGIONAL ANALYSIS
8.1. SEGMENT OVERVIEW
8.2. NORTH AMERICA
8.2.1. U.S.
8.2.2. CANADA
8.2.3. MEXICO
8.3. EUROPE
8.3.1. GERMANY
8.3.2. FRANCE
8.3.3. U.K.
8.3.4. ITALY
8.3.5. SPAIN
8.4. ASIA-PACIFIC
8.4.1. JAPAN
8.4.2. CHINA
8.4.3. INDIA
8.5. SOUTH AMERICA
8.5.1. BRAZIL
8.6. MIDDLE EAST AND AFRICA
8.6.1. UAE
8.6.2. SOUTH AFRICA
9. GLOBAL PERIPHERAL VASCULAR DEVICES MARKET-COMPETITIVE LANDSCAPE
9.1. OVERVIEW
9.2. MARKET SHARE OF KEY PLAYERS IN THE PERIPHERAL VASCULAR DEVICES MARKET
9.2.1. GLOBAL COMPANY MARKET SHARE
9.2.2. NORTH AMERICA COMPANY MARKET SHARE
9.2.3. EUROPE COMPANY MARKET SHARE
9.2.4. APAC COMPANY MARKET SHARE
9.3. COMPETITIVE SITUATIONS AND TRENDS
9.3.1. PRODUCT LAUNCHES AND DEVELOPMENTS
9.3.2. PARTNERSHIPS, COLLABORATIONS, AND AGREEMENTS
9.3.3. MERGERS & ACQUISITIONS
9.3.4. EXPANSIONS
10. COMPANY PROFILES
10.1. ANGIOSCORE INC.
10.1.1. BUSINESS OVERVIEW
10.1.2. COMPANY SNAPSHOT
10.1.3. COMPANY MARKET SHARE ANALYSIS
10.1.4. COMPANY PRODUCT PORTFOLIO
10.1.5. RECENT DEVELOPMENTS
10.1.6. SWOT ANALYSIS
10.2. ABBOTT LABORATORIES
10.2.1. BUSINESS OVERVIEW
10.2.2. COMPANY SNAPSHOT
10.2.3. COMPANY MARKET SHARE ANALYSIS
10.2.4. COMPANY PRODUCT PORTFOLIO
10.2.5. RECENT DEVELOPMENTS
10.2.6. SWOT ANALYSIS
10.3. EDWARD LIFESCIENCES CORPORATION
10.3.1. BUSINESS OVERVIEW
10.3.2. COMPANY SNAPSHOT
10.3.3. COMPANY MARKET SHARE ANALYSIS
10.3.4. COMPANY PRODUCT PORTFOLIO
10.3.5. RECENT DEVELOPMENTS
10.3.6. SWOT ANALYSIS
10.4. JUDE MEDICAL
10.4.1. BUSINESS OVERVIEW
10.4.2. COMPANY SNAPSHOT
10.4.3. COMPANY MARKET SHARE ANALYSIS
10.4.4. COMPANY PRODUCT PORTFOLIO
10.4.5. RECENT DEVELOPMENTS
10.4.6. SWOT ANALYSIS
10.5. MEDTRONIC INC.
10.5.1. BUSINESS OVERVIEW
10.5.2. COMPANY SNAPSHOT
10.5.3. COMPANY MARKET SHARE ANALYSIS
10.5.4. COMPANY PRODUCT PORTFOLIO
10.5.5. RECENT DEVELOPMENTS
10.5.6. SWOT ANALYSIS
10.6. TELEFLEX MEDICAL
10.6.1. BUSINESS OVERVIEW
10.6.2. COMPANY SNAPSHOT
10.6.3. COMPANY MARKET SHARE ANALYSIS
10.6.4. COMPANY PRODUCT PORTFOLIO
10.6.5. RECENT DEVELOPMENTS
10.6.6. SWOT ANALYSIS
10.7. VOLCANO CORPORATION
10.7.1. BUSINESS OVERVIEW
10.7.2. COMPANY SNAPSHOT
10.7.3. COMPANY MARKET SHARE ANALYSIS
10.7.4. COMPANY PRODUCT PORTFOLIO
10.7.5. RECENT DEVELOPMENTS
10.7.6. SWOT ANALYSIS
10.8. BOSTON SCIENTIFIC CORPORATION
10.8.1. BUSINESS OVERVIEW
10.8.2. COMPANY SNAPSHOT
10.8.3. COMPANY MARKET SHARE ANALYSIS
10.8.4. COMPANY PRODUCT PORTFOLIO
10.8.5. RECENT DEVELOPMENTS
10.8.6. SWOT ANALYSIS
10.9. COOK GROUP INC.
10.9.1. BUSINESS OVERVIEW
10.9.2. COMPANY SNAPSHOT
10.9.3. COMPANY MARKET SHARE ANALYSIS
10.9.4. COMPANY PRODUCT PORTFOLIO
10.9.5. RECENT DEVELOPMENTS
10.9.6. SWOT ANALYSIS
10.10. COVIDIEN
10.10.1. BUSINESS OVERVIEW
10.10.2. COMPANY SNAPSHOT
10.10.3. COMPANY MARKET SHARE ANALYSIS
10.10.4. COMPANY PRODUCT PORTFOLIO
10.10.5. RECENT DEVELOPMENTS
10.10.6. SWOT ANALYSIS
10.11. CORDIS CORPORATION
10.11.1. BUSINESS OVERVIEW
10.11.2. COMPANY SNAPSHOT
10.11.3. COMPANY MARKET SHARE ANALYSIS
10.11.4. COMPANY PRODUCT PORTFOLIO
10.11.5. RECENT DEVELOPMENTS
List of Table
1. GLOBAL PERIPHERAL VASCULAR DEVICES MARKET, BY TYPE, 2019-2032 (USD BILLION)
2. GLOBAL ANGIOPLASTY BALLOONS PERIPHERAL VASCULAR DEVICES MARKET, BY REGION, 2019-2032 (USD BILLION)
3. GLOBAL ANGIOPLASTY STENTS PERIPHERAL VASCULAR DEVICES MARKET, BY REGION, 2019-2032 (USD BILLION)
4. GLOBAL CATHETERS PERIPHERAL VASCULAR DEVICES MARKET, BY REGION, 2019-2032 (USD BILLION)
5. GLOBAL OTHERS PERIPHERAL VASCULAR DEVICES MARKET, BY REGION, 2019-2032 (USD BILLION)
6. GLOBAL PERIPHERAL VASCULAR DEVICES MARKET, BY END-USER, 2019-2032 (USD BILLION)
7. GLOBAL HOSPITALS PERIPHERAL VASCULAR DEVICES MARKET, BY REGION, 2019-2032 (USD BILLION)
8. GLOBAL AMBULATORY SURGICAL CENTERS PERIPHERAL VASCULAR DEVICES MARKET, BY REGION, 2019-2032 (USD BILLION)
9. GLOBAL SPECIALTY CLINICS PERIPHERAL VASCULAR DEVICES MARKET, BY REGION, 2019-2032 (USD BILLION)
10. GLOBAL PERIPHERAL VASCULAR DEVICES MARKET, BY REGION, 2019-2032 (USD BILLION)
11. NORTH AMERICA PERIPHERAL VASCULAR DEVICES MARKET, BY TYPE, 2019-2032 (USD BILLION)
12. NORTH AMERICA PERIPHERAL VASCULAR DEVICES MARKET, BY END-USER, 2019-2032 (USD BILLION)
13. U.S. PERIPHERAL VASCULAR DEVICES MARKET, BY TYPE, 2019-2032 (USD BILLION)
14. U.S. PERIPHERAL VASCULAR DEVICES MARKET, BY END-USER, 2019-2032 (USD BILLION)
15. CANADA PERIPHERAL VASCULAR DEVICES MARKET, BY TYPE, 2019-2032 (USD BILLION)
16. CANADA PERIPHERAL VASCULAR DEVICES MARKET, BY END-USER, 2019-2032 (USD BILLION)
17. MEXICO PERIPHERAL VASCULAR DEVICES MARKET, BY TYPE, 2019-2032 (USD BILLION)
18. MEXICO PERIPHERAL VASCULAR DEVICES MARKET, BY END-USER, 2019-2032 (USD BILLION)
19. EUROPE PERIPHERAL VASCULAR DEVICES MARKET, BY TYPE, 2019-2032 (USD BILLION)
20. EUROPE PERIPHERAL VASCULAR DEVICES MARKET, BY END-USER, 2019-2032 (USD BILLION)
21. GERMANY PERIPHERAL VASCULAR DEVICES MARKET, BY TYPE, 2019-2032 (USD BILLION)
22. GERMANY PERIPHERAL VASCULAR DEVICES MARKET, BY END-USER, 2019-2032 (USD BILLION)
23. FRANCE PERIPHERAL VASCULAR DEVICES MARKET, BY TYPE, 2019-2032 (USD BILLION)
24. FRANCE PERIPHERAL VASCULAR DEVICES MARKET, BY END-USER, 2019-2032 (USD BILLION)
25. U.K. PERIPHERAL VASCULAR DEVICES MARKET, BY TYPE, 2019-2032 (USD BILLION)
26. U.K. PERIPHERAL VASCULAR DEVICES MARKET, BY END-USER, 2019-2032 (USD BILLION)
27. ITALY PERIPHERAL VASCULAR DEVICES MARKET, BY TYPE, 2019-2032 (USD BILLION)
28. ITALY PERIPHERAL VASCULAR DEVICES MARKET, BY END-USER, 2019-2032 (USD BILLION)
29. SPAIN PERIPHERAL VASCULAR DEVICES MARKET, BY TYPE, 2019-2032 (USD BILLION)
30. SPAIN PERIPHERAL VASCULAR DEVICES MARKET, BY END-USER, 2019-2032 (USD BILLION)
31. ASIA PACIFIC PERIPHERAL VASCULAR DEVICES MARKET, BY TYPE, 2019-2032 (USD BILLION)
32. ASIA PACIFIC PERIPHERAL VASCULAR DEVICES MARKET, BY END-USER, 2019-2032 (USD BILLION)
33. JAPAN PERIPHERAL VASCULAR DEVICES MARKET, BY TYPE, 2019-2032 (USD BILLION)
34. JAPAN PERIPHERAL VASCULAR DEVICES MARKET, BY END-USER, 2019-2032 (USD BILLION)
35. CHINA PERIPHERAL VASCULAR DEVICES MARKET, BY TYPE, 2019-2032 (USD BILLION)
36. CHINA PERIPHERAL VASCULAR DEVICES MARKET, BY END-USER, 2019-2032 (USD BILLION)
37. INDIA PERIPHERAL VASCULAR DEVICES MARKET, BY TYPE, 2019-2032 (USD BILLION)
38. INDIA PERIPHERAL VASCULAR DEVICES MARKET, BY END-USER, 2019-2032 (USD BILLION)
39. SOUTH AMERICA PERIPHERAL VASCULAR DEVICES MARKET, BY TYPE, 2019-2032 (USD BILLION)
40. SOUTH AMERICA PERIPHERAL VASCULAR DEVICES MARKET, BY END-USER, 2019-2032 (USD BILLION)
41. BRAZIL PERIPHERAL VASCULAR DEVICES MARKET, BY TYPE, 2019-2032 (USD BILLION)
42. BRAZIL PERIPHERAL VASCULAR DEVICES MARKET, BY END-USER, 2019-2032 (USD BILLION)
43. MIDDLE EAST AND AFRICA PERIPHERAL VASCULAR DEVICES MARKET, BY TYPE, 2019-2032 (USD BILLION)
44. MIDDLE EAST AND AFRICA PERIPHERAL VASCULAR DEVICES MARKET, BY END-USER, 2019-2032 (USD BILLION)
45. UAE PERIPHERAL VASCULAR DEVICES MARKET, BY TYPE, 2019-2032 (USD BILLION)
46. UAE PERIPHERAL VASCULAR DEVICES MARKET, BY END-USER, 2019-2032 (USD BILLION)
47. SOUTH AFRICA PERIPHERAL VASCULAR DEVICES MARKET, BY TYPE, 2019-2032 (USD BILLION)
48. SOUTH AFRICA PERIPHERAL VASCULAR DEVICES MARKET, BY END-USER, 2019-2032 (USD BILLION)
List of Figures
1. GLOBAL PERIPHERAL VASCULAR DEVICES MARKET SEGMENTATION
2. PERIPHERAL VASCULAR DEVICES MARKET: RESEARCH METHODOLOGY
3. MARKET SIZE ESTIMATION METHODOLOGY: BOTTOM-UP APPROACH
4. MARKET SIZE ESTIMATION METHODOLOGY: TOP-DOWN APPROACH
5. DATA TRIANGULATION
6. PORTER’S FIVE FORCES ANALYSIS
7. VALUE CHAIN ANALYSIS
8. GLOBAL PERIPHERAL VASCULAR DEVICES MARKET ATTRACTIVENESS ANALYSIS BY TYPE
9. GLOBAL PERIPHERAL VASCULAR DEVICES MARKET ATTRACTIVENESS ANALYSIS BY END-USER
10. GLOBAL PERIPHERAL VASCULAR DEVICES MARKET ATTRACTIVENESS ANALYSIS BY REGION
11. GLOBAL PERIPHERAL VASCULAR DEVICES MARKET: DYNAMICS
12. GLOBAL PERIPHERAL VASCULAR DEVICES MARKET SHARE BY TYPE (2022 & 2032)
13. GLOBAL PERIPHERAL VASCULAR DEVICES MARKET SHARE BY END-USER (2022 & 2032)
14. GLOBAL PERIPHERAL VASCULAR DEVICES MARKET SHARE BY REGIONS (2022 & 2032)
15. GLOBAL PERIPHERAL VASCULAR DEVICES MARKET SHARE BY COMPANY (2022)
This study forecasts revenue at global, regional, and country levels from 2019 to 2032. The Brainy Insights has segmented the global peripheral vascular devices market based on below mentioned segments:
Global Peripheral Vascular Devices Market by Type:
Global Peripheral Vascular Devices Market by End-user:
Global Peripheral Vascular Devices Market by Region:
Research has its special purpose to undertake marketing efficiently. In this competitive scenario, businesses need information across all industry verticals; the information about customer wants, market demand, competition, industry trends, distribution channels etc. This information needs to be updated regularly because businesses operate in a dynamic environment. Our organization, The Brainy Insights incorporates scientific and systematic research procedures in order to get proper market insights and industry analysis for overall business success. The analysis consists of studying the market from a miniscule level wherein we implement statistical tools which helps us in examining the data with accuracy and precision.
Our research reports feature both; quantitative and qualitative aspects for any market. Qualitative information for any market research process are fundamental because they reveal the customer needs and wants, usage and consumption for any product/service related to a specific industry. This in turn aids the marketers/investors in knowing certain perceptions of the customers. Qualitative research can enlighten about the different product concepts and designs along with unique service offering that in turn, helps define marketing problems and generate opportunities. On the other hand, quantitative research engages with the data collection process through interviews, e-mail interactions, surveys and pilot studies. Quantitative aspects for the market research are useful to validate the hypotheses generated during qualitative research method, explore empirical patterns in the data with the help of statistical tools, and finally make the market estimations.
The Brainy Insights offers comprehensive research and analysis, based on a wide assortment of factual insights gained through interviews with CXOs and global experts and secondary data from reliable sources. Our analysts and industry specialist assume vital roles in building up statistical tools and analysis models, which are used to analyse the data and arrive at accurate insights with exceedingly informative research discoveries. The data provided by our organization have proven precious to a diverse range of companies, facilitating them to address issues such as determining which products/services are the most appealing, whether or not customers use the product in the manner anticipated, the purchasing intentions of the market and many others.
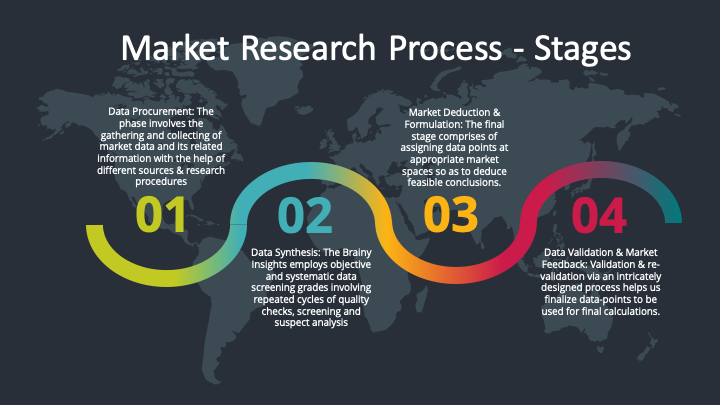
Our research methodology encompasses an idyllic combination of primary and secondary initiatives. Key phases involved in this process are listed below:
The phase involves the gathering and collecting of market data and its related information with the help of different sources & research procedures.
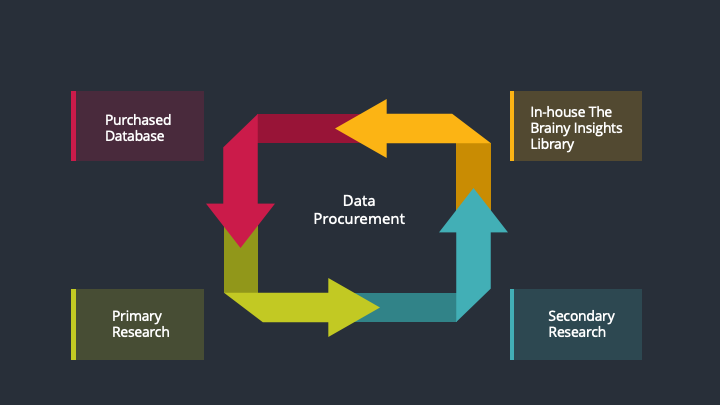
The data procurement stage involves in data gathering and collecting through various data sources.
This stage involves in extensive research. These data sources includes:
Purchased Database: Purchased databases play a crucial role in estimating the market sizes irrespective of the domain. Our purchased database includes:
Primary Research: The Brainy Insights interacts with leading companies and experts of the concerned domain to develop the analyst team’s market understanding and expertise. It improves and substantiates every single data presented in the market reports. Primary research mainly involves in telephonic interviews, E-mail interactions and face-to-face interviews with the raw material providers, manufacturers/producers, distributors, & independent consultants. The interviews that we conduct provides valuable data on market size and industry growth trends prevailing in the market. Our organization also conducts surveys with the various industry experts in order to gain overall insights of the industry/market. For instance, in healthcare industry we conduct surveys with the pharmacists, doctors, surgeons and nurses in order to gain insights and key information of a medical product/device/equipment which the customers are going to usage. Surveys are conducted in the form of questionnaire designed by our own analyst team. Surveys plays an important role in primary research because surveys helps us to identify the key target audiences of the market. Additionally, surveys helps to identify the key target audience engaged with the market. Our survey team conducts the survey by targeting the key audience, thus gaining insights from them. Based on the perspectives of the customers, this information is utilized to formulate market strategies. Moreover, market surveys helps us to understand the current competitive situation of the industry. To be precise, our survey process typically involve with the 360 analysis of the market. This analytical process begins by identifying the prospective customers for a product or service related to the market/industry to obtain data on how a product/service could fit into customers’ lives.

Secondary Research: The secondary data sources includes information published by the on-profit organizations such as World bank, WHO, company fillings, investor presentations, annual reports, national government documents, statistical databases, blogs, articles, white papers and others. From the annual report, we analyse a company’s revenue to understand the key segment and market share of that organization in a particular region. We analyse the company websites and adopt the product mapping technique which is important for deriving the segment revenue. In the product mapping method, we select and categorize the products offered by the companies catering to domain specific market, deduce the product revenue for each of the companies so as to get overall estimation of the market size. We also source data and analyses trends based on information received from supply side and demand side intermediaries in the value chain. The supply side denotes the data gathered from supplier, distributor, wholesaler and the demand side illustrates the data gathered from the end customers for respective market domain.
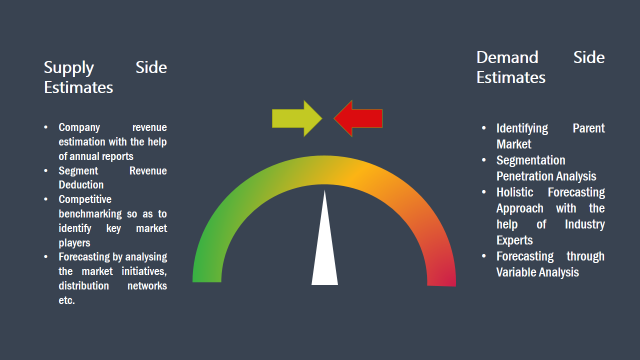
The supply side for a domain specific market is analysed by:
The demand side for the market is estimated through:
In-house Library: Apart from these third-party sources, we have our in-house library of qualitative and quantitative information. Our in-house database includes market data for various industry and domains. These data are updated on regular basis as per the changing market scenario. Our library includes, historic databases, internal audit reports and archives.
Sometimes there are instances where there is no metadata or raw data available for any domain specific market. For those cases, we use our expertise to forecast and estimate the market size in order to generate comprehensive data sets. Our analyst team adopt a robust research technique in order to produce the estimates:
Data Synthesis: This stage involves the analysis & mapping of all the information obtained from the previous step. It also involves in scrutinizing the data for any discrepancy observed while data gathering related to the market. The data is collected with consideration to the heterogeneity of sources. Robust scientific techniques are in place for synthesizing disparate data sets and provide the essential contextual information that can orient market strategies. The Brainy Insights has extensive experience in data synthesis where the data passes through various stages:
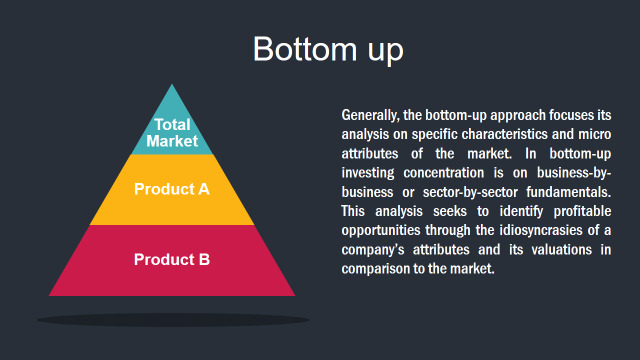
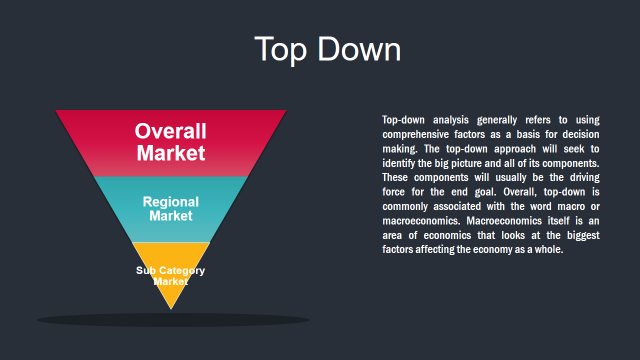
Market Deduction & Formulation: The final stage comprises of assigning data points at appropriate market spaces so as to deduce feasible conclusions. Analyst perspective & subject matter expert based holistic form of market sizing coupled with industry analysis also plays a crucial role in this stage.
This stage involves in finalization of the market size and numbers that we have collected from data integration step. With data interpolation, it is made sure that there is no gap in the market data. Successful trend analysis is done by our analysts using extrapolation techniques, which provide the best possible forecasts for the market.
Data Validation & Market Feedback: Validation is the most important step in the process. Validation & re-validation via an intricately designed process helps us finalize data-points to be used for final calculations.
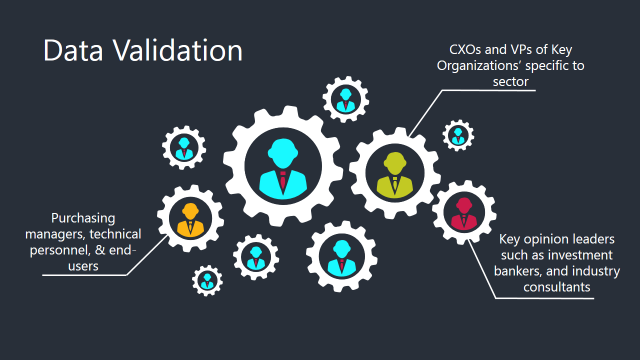
The Brainy Insights interacts with leading companies and experts of the concerned domain to develop the analyst team’s market understanding and expertise. It improves and substantiates every single data presented in the market reports. The data validation interview and discussion panels are typically composed of the most experienced industry members. The participants include, however, are not limited to:
Moreover, we always validate our data and findings through primary respondents from all the major regions we are working on.
Free Customization
Fortune 500 Clients
Free Yearly Update On Purchase Of Multi/Corporate License
Companies Served Till Date